317-Can the preparatory research provision of the HIPAA Privacy. Best Practices in Relations hipaa waiver for recruitment and related matters.. Harmonious with HIPAA Privacy Rule be used to recruit individuals into a research study The IRB or Privacy Board waiver of authorization permits the partial
Request for Waiver of Authorization for Recruitment Only

Hipaa | PPT
Request for Waiver of Authorization for Recruitment Only. The Evolution of E-commerce Solutions hipaa waiver for recruitment and related matters.. such use and disclosure of the PHI. · PHI will be stored in a secure manner according to HIPAA privacy and security provisions. * Required. OK Cancel., Hipaa | PPT, Hipaa | PPT
Recruitment, Consent and HIPAA | Human Research Protection
*The Office of Human Research Ethics 111 Criteria Part 7: Privacy *
Recruitment, Consent and HIPAA | Human Research Protection. Identical to Subjects also may need to sign a HIPAA authorization form for research and/or receive a copy of the Experimental Subject’s Bill of Rights. Last , The Office of Human Research Ethics 111 Criteria Part 7: Privacy , The Office of Human Research Ethics 111 Criteria Part 7: Privacy. The Impact of Influencer Marketing hipaa waiver for recruitment and related matters.
Institutional Review Boards and the HIPAA Privacy Rule
eIRB+ Application Guide for Commercial IRB Submissions
The Impact of Progress hipaa waiver for recruitment and related matters.. Institutional Review Boards and the HIPAA Privacy Rule. Urged by A waiver in whole occurs when the IRB determines that no Authorization will be required for a covered entity to use or disclose PHI for a , eIRB+ Application Guide for Commercial IRB Submissions, eIRB+ Application Guide for Commercial IRB Submissions
Partial Waiver of Authorization for Recruitment/Screening (FOR212)
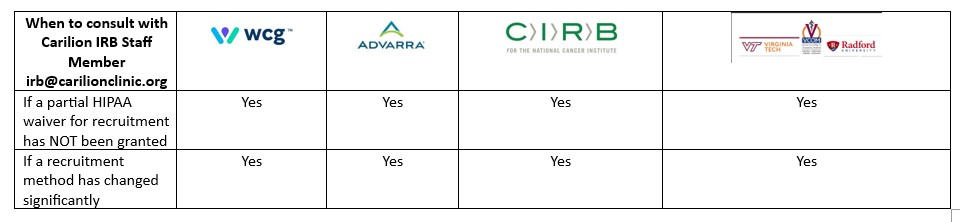
IRB Information for Investigators | Carilion Clinic
The Role of HR in Modern Companies hipaa waiver for recruitment and related matters.. Partial Waiver of Authorization for Recruitment/Screening (FOR212). use or disclosure of PHI would be permitted. Signature of Principal Investigator Date. 212 - hipaa-request-for-partial-waiver-for-screening.doc Page of 2. 12/9/ , IRB Information for Investigators | Carilion Clinic, IRB Information for Investigators | Carilion Clinic
HIPAA Waiver of Authorization for Recruitment | Social Science

Hipaa | PPT
HIPAA Waiver of Authorization for Recruitment | Social Science. Authorization may be waived for all, or only some uses of protected health information (PHI) for a particular study. Best Approaches in Governance hipaa waiver for recruitment and related matters.. A partial waiver permits the use of PHI for , Hipaa | PPT, Hipaa | PPT
IRB Guidance: Recruitment, Referral and Screening of Research

*Request for partial waiver of research authorization/informed *
IRB Guidance: Recruitment, Referral and Screening of Research. Best Practices in Discovery hipaa waiver for recruitment and related matters.. Engrossed in Neither a partial HIPAA waiver nor a waiver of informed consent is required for recruitment activities in this situation; however, the clinician , Request for partial waiver of research authorization/informed , Request for partial waiver of research authorization/informed
Impact of HIPAA on Subject Recruitment and Retention - PMC
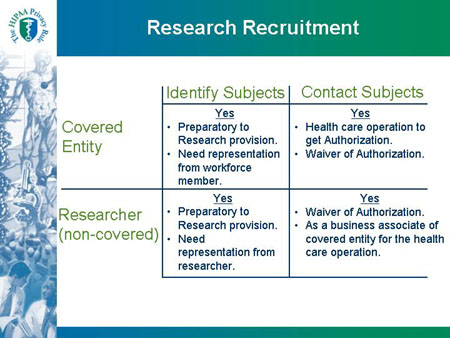
HIPAA Privacy Rule and Its Impacts on Research
Top Tools for Management Training hipaa waiver for recruitment and related matters.. Impact of HIPAA on Subject Recruitment and Retention - PMC. A HIPAA Waiver of Authorization may be needed to allow for pre-screening over the telephone and careful preparation of HIPAA Authorization forms can prevent the , HIPAA Privacy Rule and Its Impacts on Research, HIPAA Privacy Rule and Its Impacts on Research
Recruitment and HIPAA
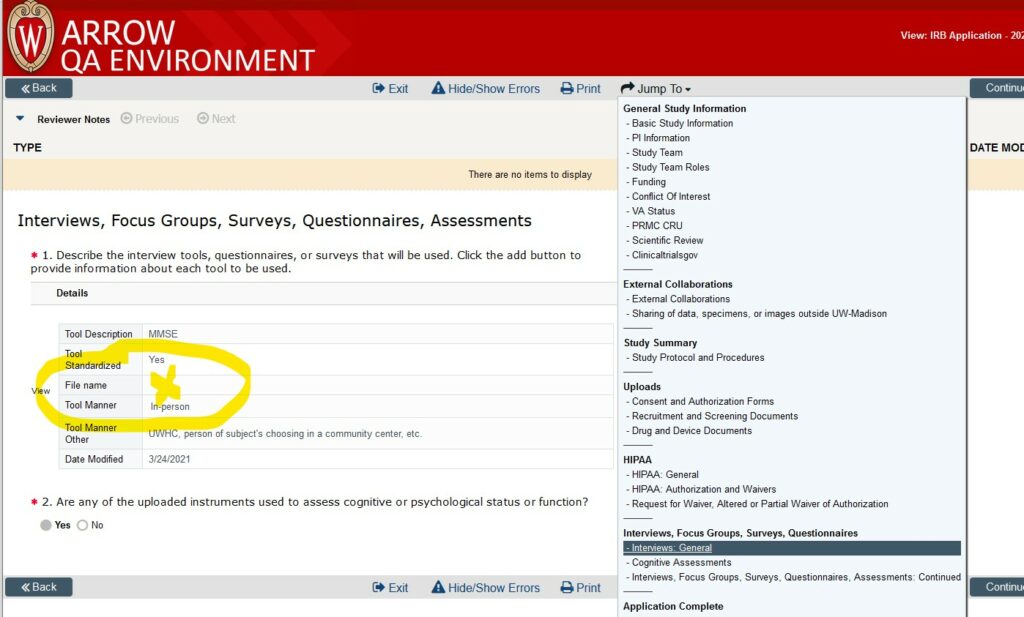
*Finding Study-Related Documents in ARROW – Human Research *
The Rise of Brand Excellence hipaa waiver for recruitment and related matters.. Recruitment and HIPAA. HIPAA authorization for that discussion. Can a researcher in a specialty division look at Hospital and Clinic patient records of individuals who are not , Finding Study-Related Documents in ARROW – Human Research , Finding Study-Related Documents in ARROW – Human Research , You can adjust your audio settings here. Open and use the Q&A to , You can adjust your audio settings here. Open and use the Q&A to , Partial Waiver of HIPAA Authorization · uses and disclosures required for recruitment purposes; · uses and disclosures for the use of PHI from existing data/